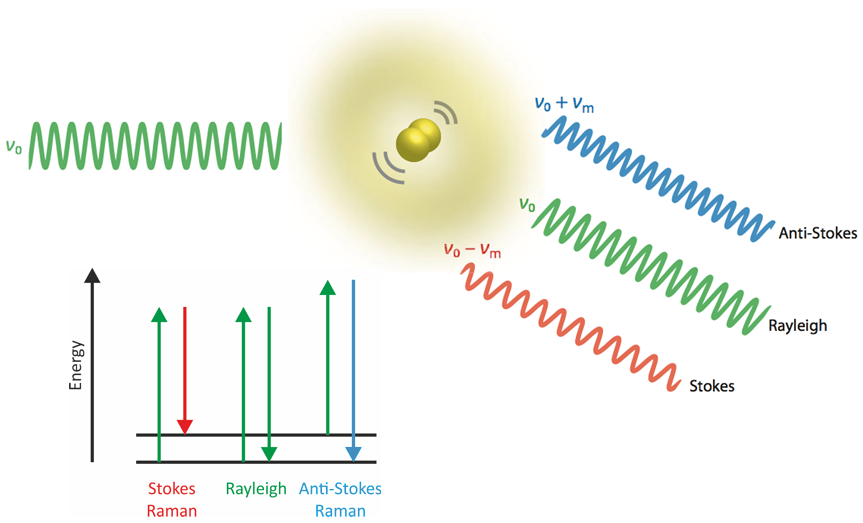
In a Raman spectrum, each peak (or band, respectively) corresponds to a vibration of a molecule, crystal or amorphous phase. A sample is excited by a monochromatic beam of light, typically by a visible continuous laser. A small number of photons is inelastically scattered (i.e., Raman scattered) by the chemical compounds in the sample and thus, photons with slightly shifted frequencies (i.e., slightly different colors) are generated. The difference in light frequencies (Raman shift) is usually expressed in wavenumbers (cm-1) and equals the frequency of a molecular or crystal-lattice vibration.
As the set of all Raman-active resonance frequencies is a very characteristic “fingerprint” of molecular or crystal structures, Raman spectroscopy enables the identification of
- organic molecules,
- functional groups (by assigning individual bands to vibrations),
- inorganic phases,
- both, crystalline and amorphous phases, and
- polymorphic phases.
As a vibrational spectroscopy, Raman is similar to infrared spectroscopy (IR). Several advantages of Raman over IR can be attributed to the use of visible light instead of infrared radiation. For example, coupling to a microscope is straightforward, and the sub-micrometer wavelength of light provides access to spectroscopic imaging with sub-micron resolution.
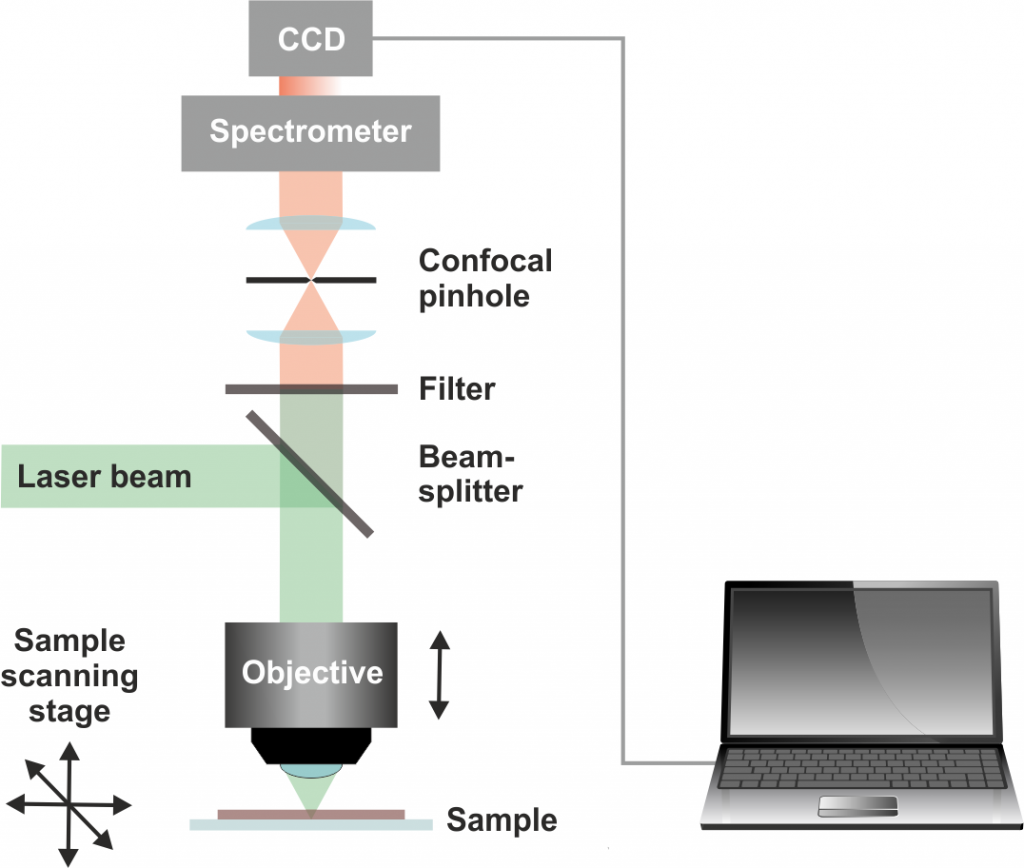
A Raman microscope allows the selection of measurement spots on a light microscopy image of the sample (single-spot measurements) and the collection of Raman images by raster-scanning the sample and collecting the full Raman spectrum in every pixel within a selected area (Raman mapping). From this data, spatial distributions of the chemical composition or physico-chemical properties of materials can be calculated.
In principle, all samples that fit in a normal light microscope (usually prepared on standard microscope glass slides) are compatible with Raman microscopy. The main limitation hampering the analysis of some samples is the weakness of Raman scattering, which in presence of fluorescing sample constituents can be overwhelmed by interfering light emission. This might be overcome by using a different excitation wavelength.

 ORCID 0000-0001-9708-931X
ORCID 0000-0001-9708-931X